At the forefront of cancer therapy
Introducing the BRiTE (Brain Bispecific T cell Engager) platform, a cutting-edge bispecific antibody technology developed to target and treat intracerebral malignancies such as gliomas.
Spearheaded by a distinguished team of researchers at Duke University, the BRiTE platform is poised to revolutionize the treatment of aggressive brain tumors through its unique and highly effective approach.
Preclinical studies of the BRiTE platform underscore its potential as a highly effective, targeted and safe treatment for gliomas. The ability of BRiTE to activate and direct T cells to specifically target and lyse glioma cells, coupled with its innovative delivery mechanism, positions it as a promising therapeutic option for patients with newly diagnosed or recurrent intracerebral malignancies.
The Science behind BRiTE
The BRiTE platform utilizes a combination of specifically manipulated T cells with bispecific antibodies to enhance the transport of bispecific antibodies to tissues of interest, including the brain. For glioblastoma, the bispecific antibody simultaneously targets the EGFRvIII mutation, a variant commonly found in gliomas, and the CD3 receptor on T cells. Localized at the tumor site, this targeting redirects the full repertoire of T cells at the tumor site to lyse tumor cells, facilitating precise and potent anti-tumor activity.
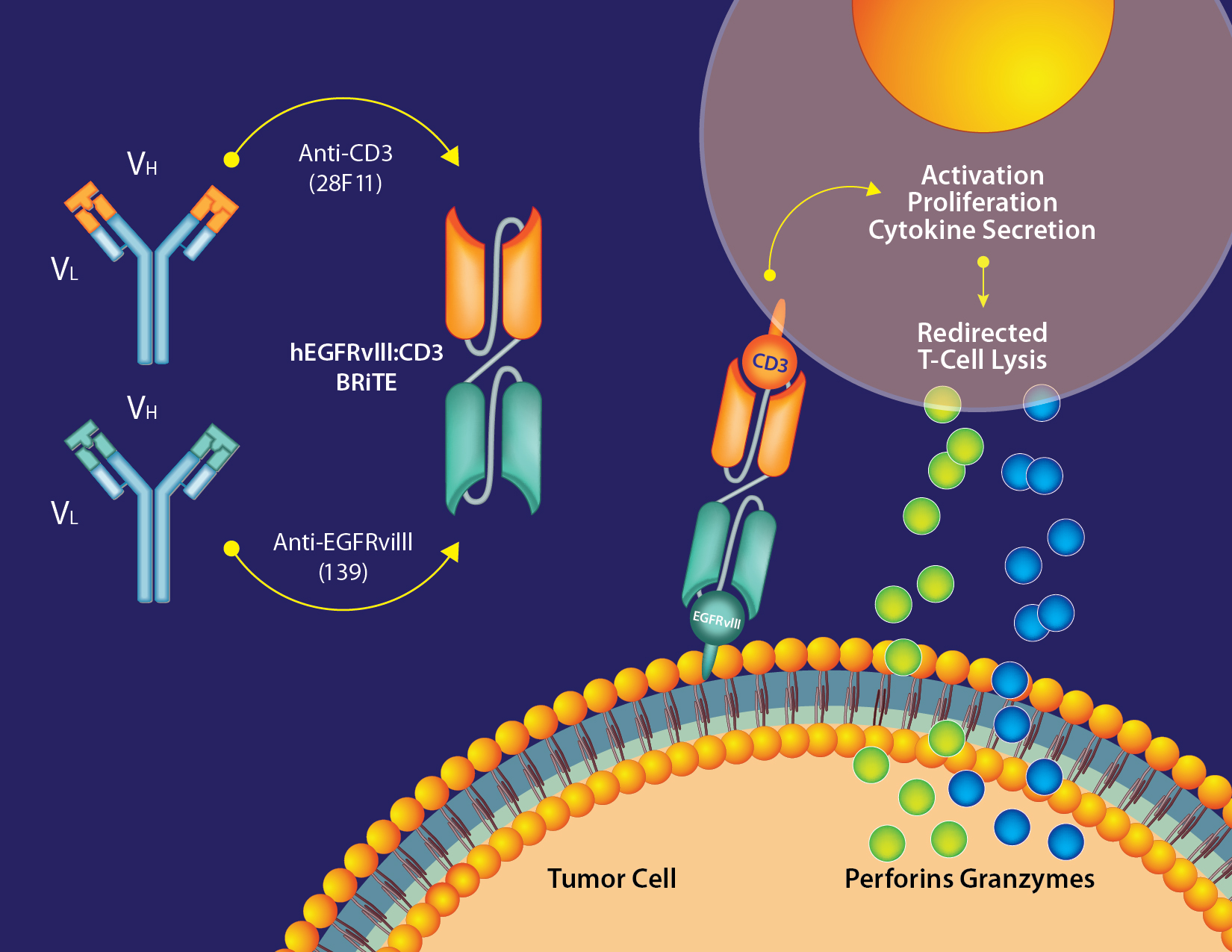
Targeted therapy with dual mechanism of action

Recognition
- EGFRvIII (and other Tumor Associated Antigens [TAA]) Targeting: The antibody specifically binds to the EGFRvIII mutation, which is highly prevalent in malignant gliomas, ensuring that the treatment is localized and effective.
Engagement
- CD3 Engagement: By binding to CD3 on T cells, BRiTE activates these immune cells and directs them to attack the tumor cells, harnessing the body’s own defense mechanisms.
Cytotoxic Effect
- After binding with the CD3 subunit on T-cells and the TAA on the tumor cell (EGFRvIII) a cytolytic synapse is formed.
- Importantly, this bypasses the need for MHC presentation, directly triggering activation signaling leading to the release of the pore-forming perforin and cytotoxic granzyme-B (GzmB). This results in apoptosis of the target tumor cell

